Septation
General Description
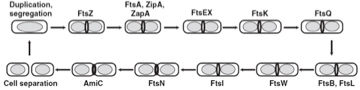
Invagination of the inner and outer membranes, as well as the cell wall that occurs to separate daughter cells. There are 4 main steps:
- Selection of the division site
- Recruitment of the septal proteins to the division site and formation of the ring
- Constriction of the membrane and cell wall
- Separation of the 2 daughter cells
Components
FtsZ(Z ring)[5], FtsA, FtsK[6][7], FtsN[8], FtsI, FtsE, FtsX, FtsQ[9], etc.
- Absolutely all septation events include FtsZ[10].
In order to ensure the chromosome doesn't get guillotined, TopoIV and the XerCD recombinase are also localized to the septa, via FtsK, but they are not involved directly in the membrane closure.
Some of the experiments that helped to order the components of this recruitment pathway can be found in Chen et al. (1999)[12], Weiss et al. (1999)[13], Ghigo et al. (1999)[14], Wang and Lutkenhaus (1998)[7], and Mercer and Weiss (2002)[15].
FtsI info[16]
Function
Regulation
Z ring formation can be inhibited by SulA (via recA-dependent SOS response) or by the action of the Min system[17][10]. SOS gets triggered in response to DNA damage. sulA is one of many genes whose expression gets upregulated upon LexA repressor cleavage[18]. The SulA protein directly binds to monomeric FtsZ and inhibits its polymerization[19].
The FtsK protein couples septation and chromosome segregation, however only the N-terminal domain of FtsK functions in cell division[20].
Cell Biology
Mutations can occur which cause cells to grow as septated chains instead of filaments. Some of these mutations are:
- xerC
- ftsK (its CTD must be present at the septum for correct functioning of Xer at dif [21], mutations that suppress the ftsK44 chaining phenotype are described in Geissler and Margolin (2005)[11].
- amiC
Experimental Resources
ftsK deletions and septal ring component plasmids are listed in Geissler and Margolin (2005)[22].
Comparison with other organisms
Notes
References
See Help:References for how to manage references in EcoliWiki.
- ↑ Pla, J et al. (1991) Preferential cytoplasmic location of FtsZ, a protein essential for Escherichia coli septation. Mol. Microbiol. 5 1681-6 PubMed
- ↑ Addinall, SG et al. (1997) Temperature shift experiments with an ftsZ84(Ts) strain reveal rapid dynamics of FtsZ localization and indicate that the Z ring is required throughout septation and cannot reoccupy division sites once constriction has initiated. J. Bacteriol. 179 4277-84 PubMed
- ↑ de Boer, P et al. (1992) The essential bacterial cell-division protein FtsZ is a GTPase. Nature 359 254-6 PubMed
- ↑ RayChaudhuri, D & Park, JT (1992) Escherichia coli cell-division gene ftsZ encodes a novel GTP-binding protein. Nature 359 251-4 PubMed
- ↑ Dai, K & Lutkenhaus, J (1991) ftsZ is an essential cell division gene in Escherichia coli. J. Bacteriol. 173 3500-6 PubMed
- ↑ Yu, XC et al. (1998) Localization of cell division protein FtsK to the Escherichia coli septum and identification of a potential N-terminal targeting domain. J. Bacteriol. 180 1296-304 PubMed
- ↑ 7.0 7.1 Wang, L & Lutkenhaus, J (1998) FtsK is an essential cell division protein that is localized to the septum and induced as part of the SOS response. Mol. Microbiol. 29 731-40 PubMed
- ↑ Addinall, SG et al. (1997) FtsN, a late recruit to the septum in Escherichia coli. Mol. Microbiol. 25 303-9 PubMed
- ↑ Chen, JC et al. (1999) Septal localization of FtsQ, an essential cell division protein in Escherichia coli. J. Bacteriol. 181 521-30 PubMed
- ↑ 10.0 10.1 Bi, E & Lutkenhaus, J (1993) Cell division inhibitors SulA and MinCD prevent formation of the FtsZ ring. J. Bacteriol. 175 1118-25 PubMed
- ↑ 11.0 11.1 Geissler, B & Margolin, W (2005) Evidence for functional overlap among multiple bacterial cell division proteins: compensating for the loss of FtsK. Mol. Microbiol. 58 596-612 PubMed
- ↑ Chen, JC & Beckwith, J (2001) FtsQ, FtsL and FtsI require FtsK, but not FtsN, for co-localization with FtsZ during Escherichia coli cell division. Mol. Microbiol. 42 395-413 PubMed
- ↑ Weiss, DS et al. (1999) Localization of FtsI (PBP3) to the septal ring requires its membrane anchor, the Z ring, FtsA, FtsQ, and FtsL. J. Bacteriol. 181 508-20 PubMed
- ↑ Ghigo, JM et al. (1999) Localization of FtsL to the Escherichia coli septal ring. Mol. Microbiol. 31 725-37 PubMed
- ↑ Mercer, KL & Weiss, DS (2002) The Escherichia coli cell division protein FtsW is required to recruit its cognate transpeptidase, FtsI (PBP3), to the division site. J. Bacteriol. 184 904-12 PubMed
- ↑ Nguyen-Distèche, M et al. (1998) The structure and function of Escherichia coli penicillin-binding protein 3. Cell. Mol. Life Sci. 54 309-16 PubMed
- ↑ Shiomi, D & Margolin, W (2007) The C-terminal domain of MinC inhibits assembly of the Z ring in Escherichia coli. J. Bacteriol. 189 236-43 PubMed
- ↑ Hill, TM et al. (1997) sfi-independent filamentation in Escherichia coli Is lexA dependent and requires DNA damage for induction. J. Bacteriol. 179 1931-9 PubMed
- ↑ Cordell, SC et al. (2003) Crystal structure of the SOS cell division inhibitor SulA and in complex with FtsZ. Proc. Natl. Acad. Sci. U.S.A. 100 7889-94 PubMed
- ↑ Draper, GC et al. (1998) Only the N-terminal domain of FtsK functions in cell division. J. Bacteriol. 180 4621-7 PubMed
- ↑ Barre, FX et al. (2000) FtsK functions in the processing of a Holliday junction intermediate during bacterial chromosome segregation. Genes Dev. 14 2976-88 PubMed
- ↑ Ohman, JL Jr (1992) Allergen immunotherapy. Review of efficacy and current practice. Med. Clin. North Am. 76 977-91 PubMed